- Get link
- X
- Other Apps
Vaccines build your bodys immune system so that in the future its able to fight against a specific disease. Life-saving lessons from worlds deadliest outbreaks The first test of an experimental coronavirus vaccine began on Monday at.
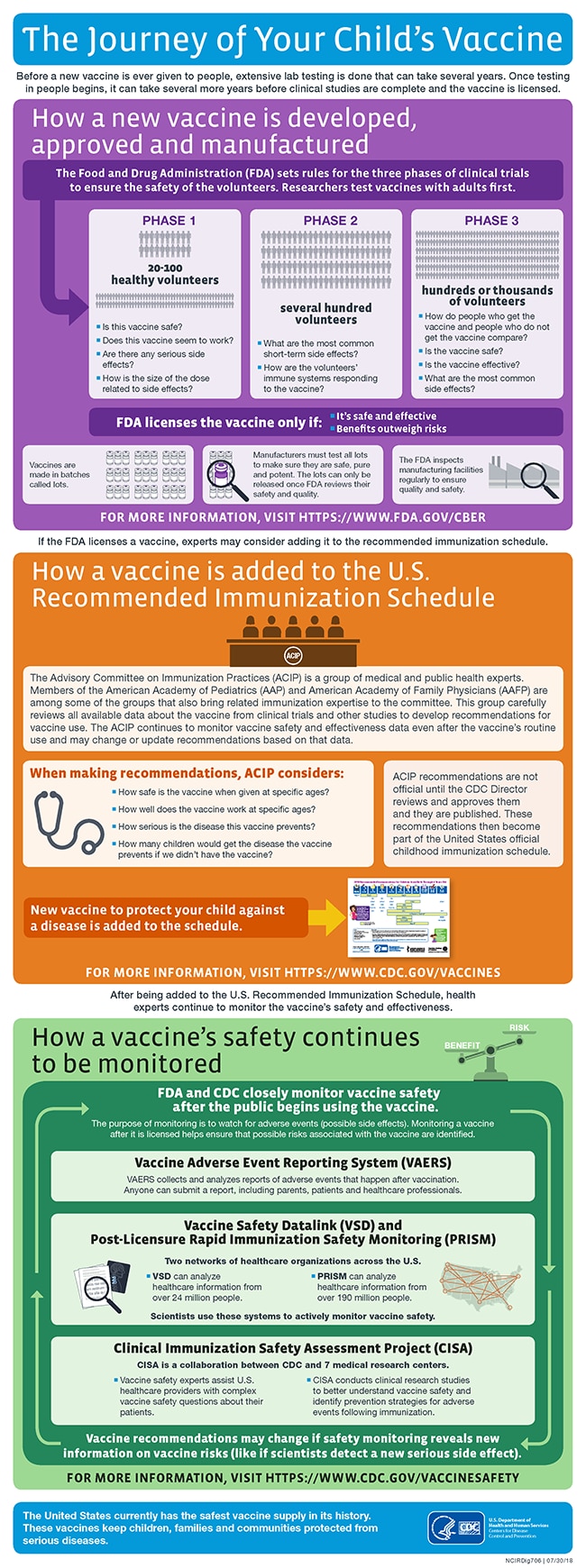 The Journey Of Your Child S Vaccine Infographic Cdc
The Journey Of Your Child S Vaccine Infographic Cdc
Vaccine manufacturers must test all lots of a vaccine to make sure they are safe pure and potent.
Are vaccines tested for safety. All vaccines go through clinical trials to test safety and effectiveness. The TGA assesses vaccines before they can be used in Australia The TGA rigorously assesses vaccines for safety quality and efficacy before they can be used in Australia. There are three phases of clinical trials.
Every authorized or approved vaccine goes through safety testing including. Vaccine lots cannot be distributed until released by FDA. In many regards the evaluation of vaccines is the same as the process for.
In Australia vaccines must pass strict safety testing before the Therapeutic Goods Administration TGA will register them for use. For the COVID-19 vaccine the FDA set high safety standards for vaccine developers to meet. Vaccines are manufactured in batches called lots and vaccine manufacturers must test all lots of a vaccine to make sure they are safe pure and potent.
Cal testing of vaccines to ensure their safety and efficacy leading to safe vaccines that have saved millions of lives. Testing and evaluation of the vaccine before its licensed by the Food and Drug Administration FDA and recommended for use by the Centers for Disease Control and Prevention CDC Monitoring the vaccines safety after it is recommended for infants children or adults. From the Spanish flu to coronavirus.
After a vaccine is licenced it continues to be monitored as part of a post-licensure monitoring of vaccines. Lessons learned include that all batches of vaccines must be tested for. This is not true they have all been put through standard safety testing.
Approval of vaccines can take up to 10 years. Before a new vaccine is given to people a lot of testing is done in a lab. Then its tested in people in clinical trials to make sure its safe and effective.
The exhaustive amount of scientific evidence confirming that vaccines are safe should ease anyones concerns about how theyre made and tested. CLAIM 1- Allthe vaccinesare consideredexperimental According to thepostallvaccines areconsidered experimental. These vaccines were held to the same standards used to ensure the safety of any approved vaccine.
The flu vaccine is no exception. It often takes many years for a vaccine to make it through the trials and tests it. Why vaccines are safe All vaccines are thoroughly tested to make sure they will not harm you or your child.
But many vaccines that appear to be safe and induce protective immune responses in animals fail in human studies. This infographic from the National Institutes of Health shows the four phases a vaccine goes through before it is released to the public. Also the UK regulator the Medicines and Healthcare products Regulatory Agency MHRAmonitors vaccines to detect any possible signals of adverse events.
The manufacturer of the vaccine may continue to test for safety efficacy and other potential uses called Phase IV Trials. Research and testing is an essential part of developing safe and effective vaccines. A leading pediatrician and vaccine expert answers parents concerns about the safety of the Covid-19 vaccine for teens between the ages of 12 and 15.
Vaccines receive the same high level of scrutiny as other prescription medicines and related therapeutic goods. Vaccine lots cannot be distributed until released by FDA. Once a vaccine is licensed FDA regularly inspects vaccine manufacturing facilities to make sure they are following strict regulations.
The bottom line is that vaccines are extensively and carefully tested for safety and that vaccine safety is shown by many many studies from a variety of sources reinforcing each other and all pointing to the same result serious problems from vaccines are possible but extremely rare. The scientific evaluation of vaccines involves animal testing human clinical trials and post-approval surveillance. Only vaccine candidates that are very promising in preclinical testing move forward into phase I clinical trials.
Preclinical studies are important for eliminating potential vaccines that are either toxic or do not induce protective immune responses. Empirical experience including evaluation of vaccine-associated adverse events indi-cates the importance of thoroughly assessing safety of vaccine preparations before licensing and widespread use.
 Covid 19 Vaccines Development Evaluation Approval And Monitoring European Medicines Agency
Covid 19 Vaccines Development Evaluation Approval And Monitoring European Medicines Agency
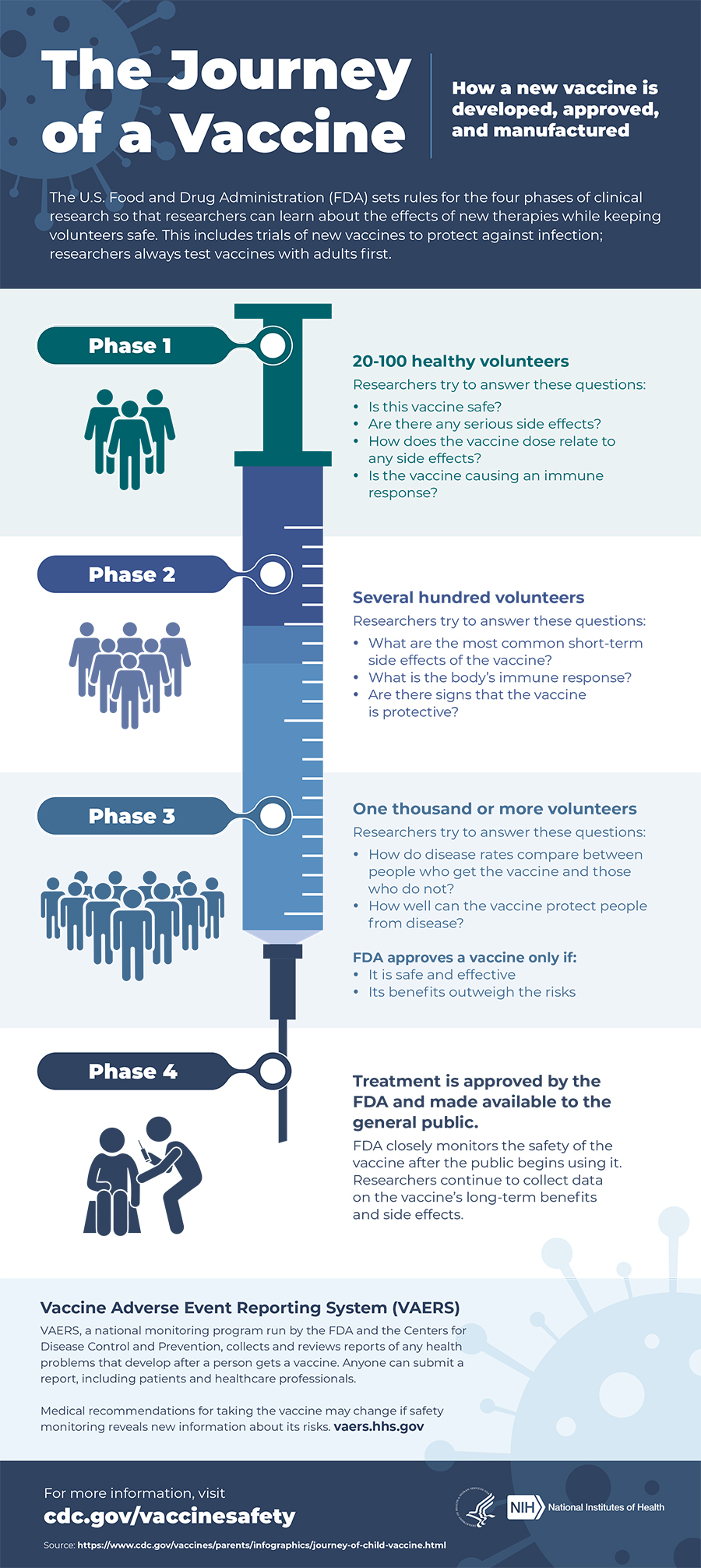 Is The Covid 19 Vaccine Safe Johns Hopkins Medicine
Is The Covid 19 Vaccine Safe Johns Hopkins Medicine
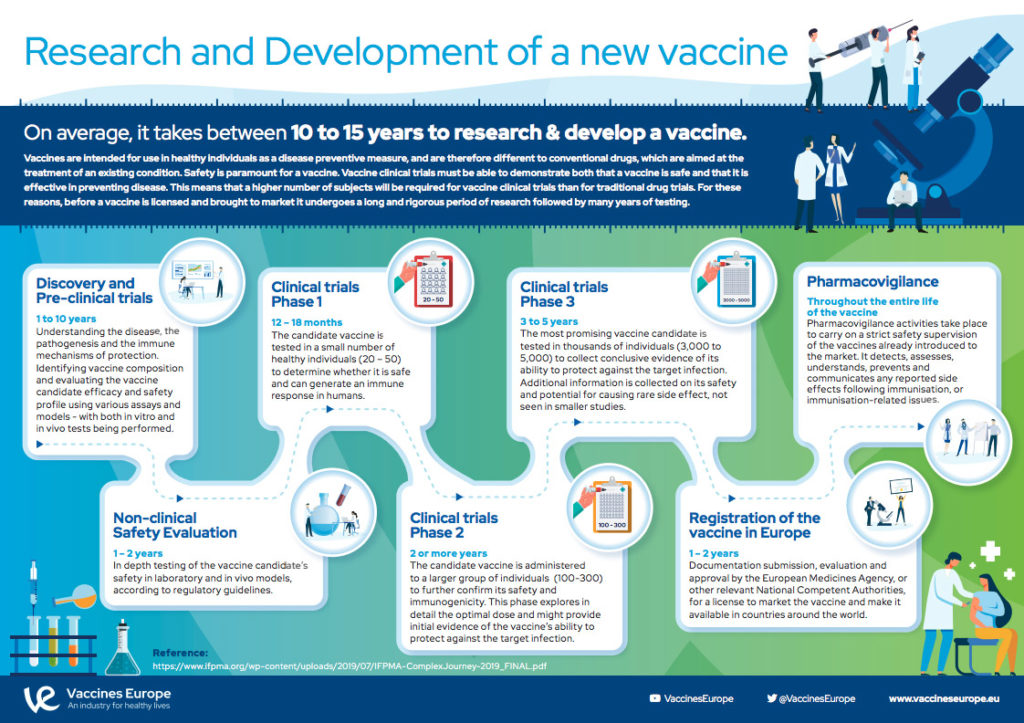 How Are Vaccines Developed Vaccines Europe
How Are Vaccines Developed Vaccines Europe
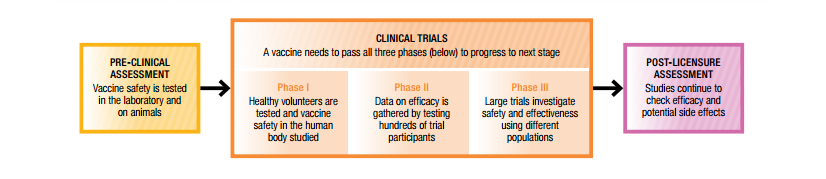 5 How Are Vaccines Shown To Be Safe Australian Academy Of Science
5 How Are Vaccines Shown To Be Safe Australian Academy Of Science
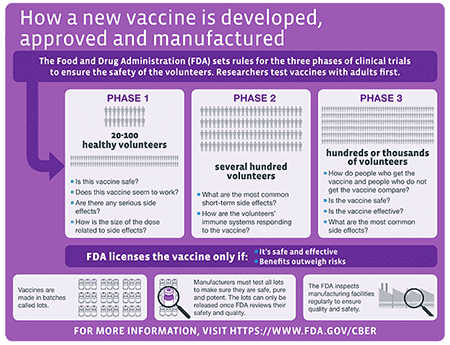 Ensuring The Safety Of Vaccines In The United States Cdc
Ensuring The Safety Of Vaccines In The United States Cdc
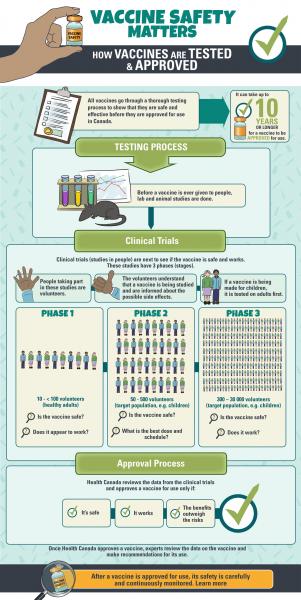 Testing Approval And Monitoring Immunize Bc
Testing Approval And Monitoring Immunize Bc
Just The Vax Please Another Vaccine Myth Busted This Time On Safety Testing Healthmap
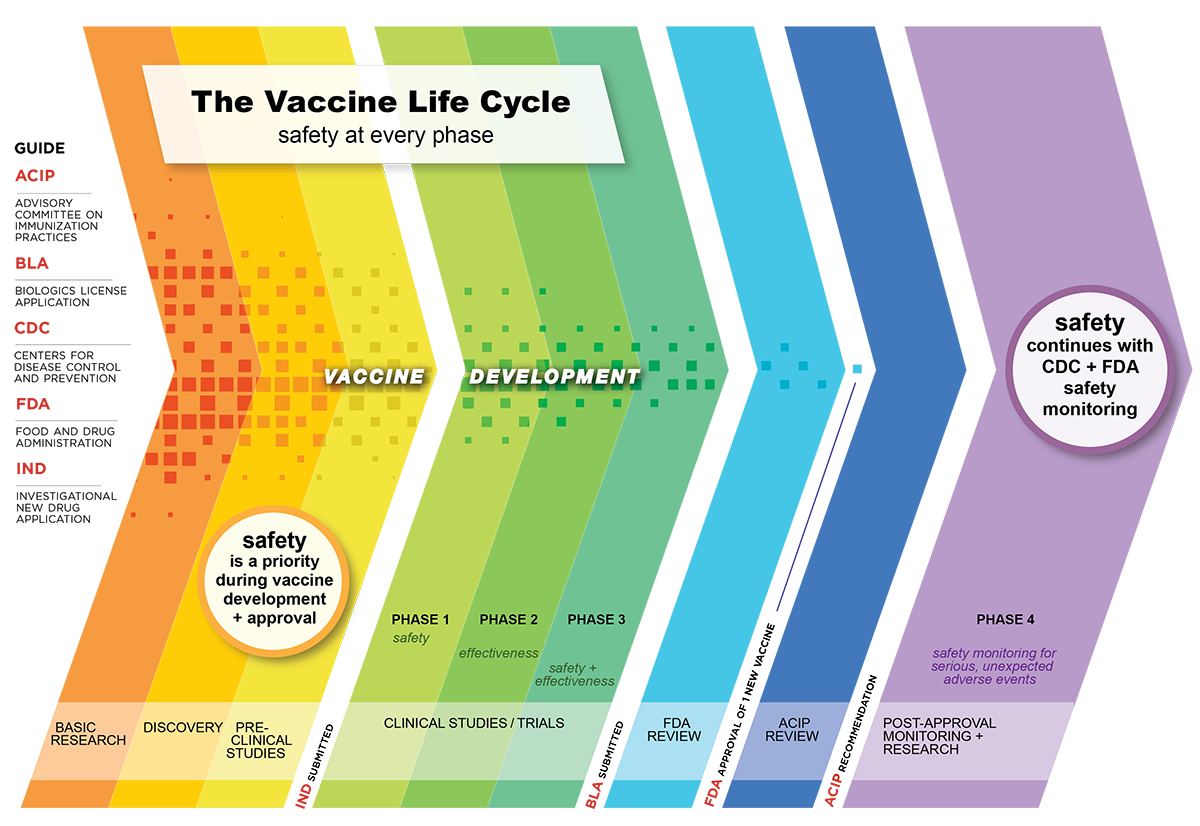 U S Vaccine Safety Overview History And How It Works Cdc
U S Vaccine Safety Overview History And How It Works Cdc
 Vaccine Science Safety National Foundation For Infectious Diseases
Vaccine Science Safety National Foundation For Infectious Diseases
 Ensuring Vaccine Safety Science
Ensuring Vaccine Safety Science
 How Are Vaccines Shown To Be Safe Fact Sheet Australian Government Department Of Health
How Are Vaccines Shown To Be Safe Fact Sheet Australian Government Department Of Health
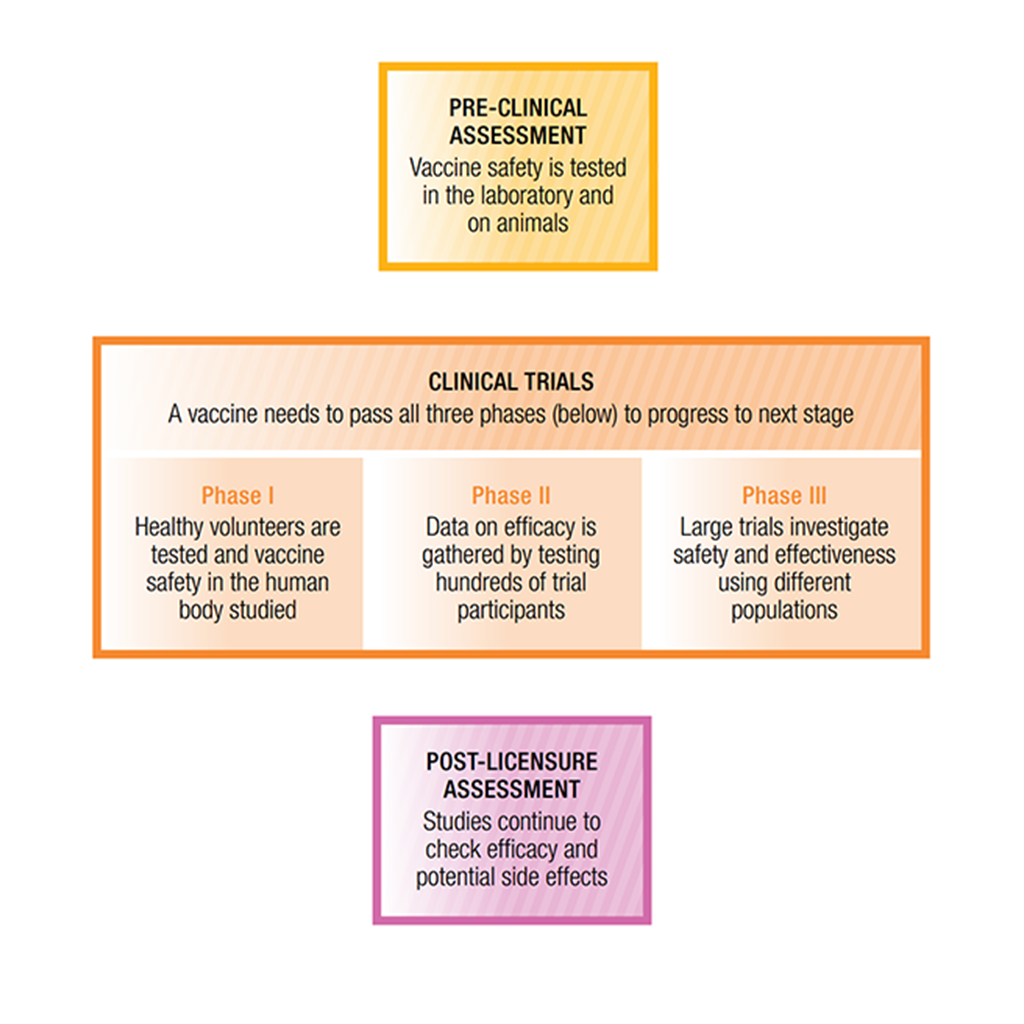

Comments
Post a Comment